Corrosion is a critical issue in the oil, gas, petrochemical, and energy industries, as it can significantly impact equipment performance, safety, and overall operational efficiency. The corrosion mechanisms that occur in these sectors vary in severity and impact, making it crucial to understand the causes, classifications, and preventive measures.
This article will explore corrosion engineering concepts and various corrosion protection methods, explaining how advanced solutions such as PetroSync can help manage these challenges effectively.
What is A Corrosion?
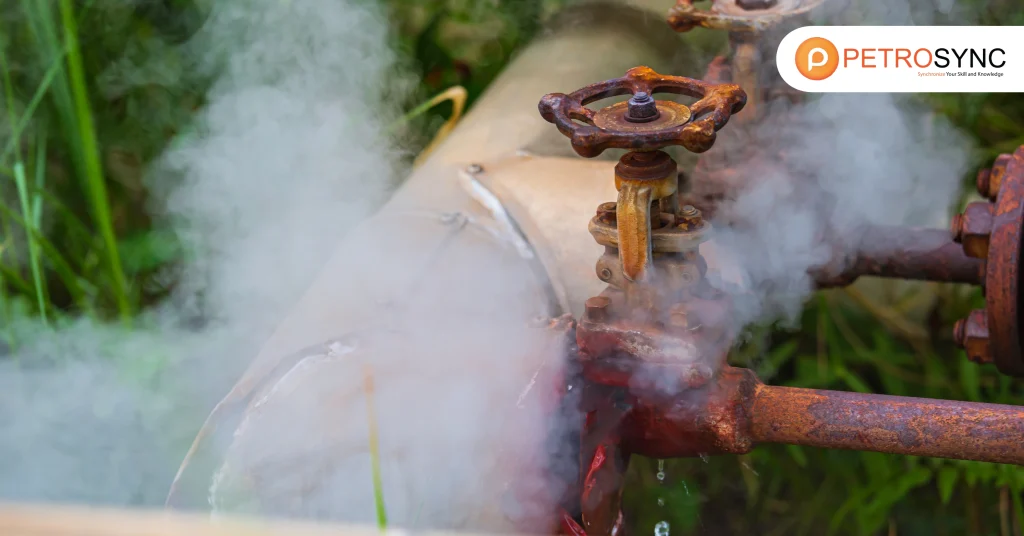
Corrosion refers to the gradual destruction or deterioration of materials, usually metals, due to chemical reactions with their environment. In industrial settings, this often involves the reaction of metals with moisture, air, or chemicals, leading to the formation of rust or other corrosion products.
The process is electrochemical, typically involving an anode, cathode, and electrolyte, with electrons flowing from the anode to the cathode through an external circuit. Corrosion is often accelerated by harsh environmental factors such as high humidity, exposure to saltwater, or the presence of acidic or basic chemicals.
Its effects on industrial assets, especially in the oil and gas sector, can lead to equipment failure, downtime, and costly repairs. Therefore, understanding the corrosion mechanisms and different types of corrosion is vital for maintaining the integrity and longevity of assets.
What is The Classification of Corrosion?
Corrosion can be classified in various ways based on its mechanism, appearance, or the material involved. The most common classifications include:
1. Electrochemical Corrosion:
This occurs when two different metals are in contact with an electrolyte, leading to electron flow and the degradation of one metal.
2. Galvanic Corrosion:
A subset of electrochemical corrosion, this happens when two different metals with different electrochemical potentials are in contact, causing one to corrode more quickly than the other.
3. Crevice Corrosion:
Occurs in confined spaces where the electrolyte becomes stagnant, such as in joints, bolts, or welds, leading to accelerated corrosion in those areas.
4. Pitting Corrosion:
This involves the formation of small, localized pits on the metal surface, which can lead to severe structural damage if not addressed.
5. Uniform Corrosion:
The most common form, uniform corrosion occurs evenly across the surface, often resulting in a gradual thinning of the material.
Other forms of corrosion include stress corrosion cracking, intergranular corrosion, and hydrogen embrittlement, each of which has unique causes and impacts on materials.
What are The Four Main Types of Corrosion?
Understanding the four main types of corrosion is crucial for industrial applications. These types include:
1. Uniform Corrosion:
As the name suggests, uniform corrosion affects the entire surface evenly, typically due to exposure to a corrosive environment. It is the most predictable form and often occurs in pipes, tanks, and vessels.
2. Pitting Corrosion:
Pitting corrosion is localized and results in the formation of small holes or pits in the material. It is often caused by the presence of chlorides or acidic environments, leading to the breakdown of the metal surface.
3. Crevice Corrosion:
This form occurs in areas where the electrolyte is trapped in small spaces, such as joints, bolts, or cracks. The lack of oxygen and the concentration of corrosive agents accelerate the corrosion process in these crevices.
4. Galvanic Corrosion:
When two dissimilar metals are in contact in the presence of an electrolyte, one metal may corrode faster than the other. The more anodic metal corrodes, while the cathodic metal remains protected. This is commonly seen in installations where metals like steel and aluminum are in contact.
What are The 10 Types of Corrosion?
While the four main types of corrosion are essential to understand, there are several other types that can pose unique challenges in industrial settings. These include:
1. Stress Corrosion Cracking (SCC):
This occurs when a material is subjected to both tensile stress and a corrosive environment, leading to the formation of cracks that can propagate over time.
2. Intergranular Corrosion:
This type occurs along the grain boundaries of a metal, often due to improper heat treatment or exposure to certain chemicals, causing the material to weaken.
3. Hydrogen Embrittlement:
When hydrogen atoms are absorbed into a metal, they can cause the material to become brittle and crack under stress, especially in high-pressure environments like oil and gas pipelines.
4. Erosion Corrosion:
This type results from the combined effects of mechanical wear and corrosion, often seen in high-velocity flows of liquids or gases that can erode the protective oxide layer on materials.
5. Microbiologically Influenced Corrosion (MIC):
This form occurs due to the presence of microorganisms such as bacteria, fungi, or algae, which accelerate corrosion through their metabolic processes.
6. Fretting Corrosion:
Caused by the relative motion between two materials in contact under load, fretting corrosion results in surface damage and increased wear.
7. Galvanic Corrosion:
As previously mentioned, this occurs when two dissimilar metals are in contact with an electrolyte, leading to corrosion of the more anodic metal.
8. Selective Leaching (Dezincification):
This occurs in brass alloys when zinc is selectively leached out of the alloy, leaving behind a porous copper structure that can fail under stress.
9. Burnout Corrosion:
Often seen in equipment subjected to high temperatures, burnout corrosion occurs when a metal’s protective oxide layer is damaged due to heat, exposing the material to accelerated corrosion.
10. Bimetallic Corrosion:
This occurs when two different metals are connected, causing the more reactive metal to corrode at a faster rate.
What are The 8 Forms of Corrosion?
The forms of corrosion are often classified based on their appearance or behavior, and they include:
General Corrosion: A uniform, widespread form of corrosion that occurs evenly across a material’s surface.
Pitting Corrosion: Localized corrosion leading to the formation of small pits on the surface.
Crevice Corrosion: Occurs in confined spaces, such as bolted joints or cracks, where the electrolyte stagnates.
Galvanic Corrosion: A type of corrosion that happens when two dissimilar metals are in contact with an electrolyte.
Stress Corrosion Cracking (SCC): The formation of cracks under stress in a corrosive environment.
Intergranular Corrosion: Corrosion along the grain boundaries of metals.
Hydrogen Embrittlement: Caused by the absorption of hydrogen, making metals brittle and prone to cracking.
Fretting Corrosion: Caused by the relative motion between two materials in contact under load.
Unlocking the Power of PetroSync: The Ultimate Solution for Effective Corrosion Management
Managing corrosion is a critical aspect of maintaining operational efficiency in industries like oil and gas. PetroSync offers a comprehensive suite of solutions designed to monitor, manage, and mitigate corrosion risks effectively. These solutions include advanced corrosion monitoring and corrosion inspection systems.
Which provide real-time data on corrosion rates, enabling proactive maintenance and early detection of potential issues. With predictive analytics powered by machine learning and AI, PetroSync helps businesses forecast corrosion risks and optimize maintenance schedules based on historical data. In addition to monitoring and predictive tools.
PetroSync offers a range of corrosion protection materials, including inhibitors and protective coatings, to extend the lifespan of equipment and minimize the impact of corrosion. The company also specializes in corrosion materials management, ensuring the selection of appropriate materials suited to specific environmental conditions.
Which enhances the durability of critical infrastructure. Furthermore, PetroSync provides specialized corrosion engineer training, equipping teams with the skills and knowledge needed to implement effective corrosion management strategies and maintain the integrity of assets across industries.







